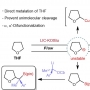
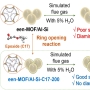
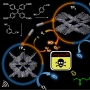
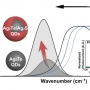
Copper-based catalysts have attracted enormous attention due to their high selectivity for C2+ products during the electrochemical reduction of CO2 (CO2RR). In particular, grain boundaries on the catalysts contribute to the generation of various Cu coordination environments, which have been found essential for C—C coupling. However, smooth-surfaced Cu2O nanocrystals generally lack the ability for the surface reorganization to form multiple grain boundaries and desired Cu undercoordination sites. Flow chemistry armed with the unparalleled ability to mix reaction mixture can achieve a very high concentration of unstable reaction intermediates, which in turn are used up rapidly to lead to kinetics-driven nanocrystal growth. Herein, the synthesis of a unique hierarchical structure of Cu2O with numerous steps (h-Cu2O ONS) via flow chemistry-assisted modulation of nanocrystal growth kinetics is reported. The surface of h-Cu2O ONS underwent rapid surface reconstruction under CO2RR conditions to exhibit multiple heterointerfaces between Cu2O and Cu phases, setting the preferable condition to facilitate C—C bond formation. Notably, the h-Cu2O ONS obtained the increased C2H4 Faradaic efficiency from 31.9% to 43.5% during electrocatalysis concurrent with the morphological reorganization, showing the role of the stepped surface. Also, the h-Cu2O ONS demonstrated a 3.8-fold higher ethylene production rate as compared to the Cu2O nanocube.

https://onlinelibrary.wiley.com/doi/10.1002/smtd.202200074
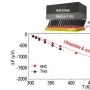 Thermopower of Molecular Junction in Harsh Thermal Environments
Thermopower of Molecular Junction in Harsh Thermal Environments
 Chemical Fields: Directing Atom Migration in the Multiphasic ...
Chemical Fields: Directing Atom Migration in the Multiphasic ...