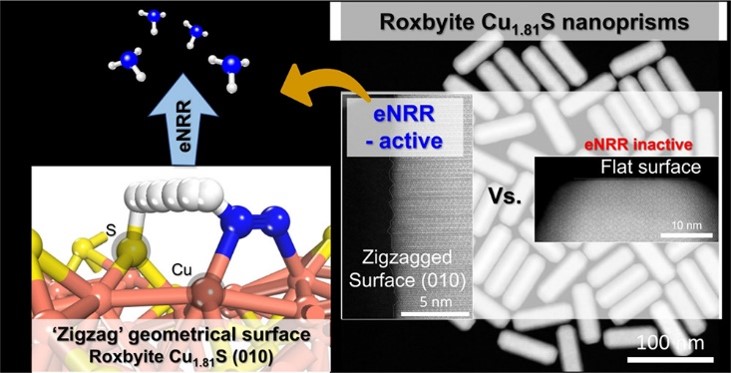
Understanding catalytic-conversion determinants will blueprint an efficient electrocatalyst design for electrochemical nitrogen reduction. In metal chalcogenide-based catalysts, metal-site nitrogen adsorption initiates nitrogen fixation, and successive hydrogen supply from nearby chalcogen sites hydrogenates the nitrogen to ammonia. However, surface geometry-dependent reaction kinetics are rarely studied because the reaction is very fast. Here, we investigate the relationship between catalyst geometrical features and their electrochemical nitrogen reduction kinetics using surface atomic geometry-regulated copper sulfide (Cu1.81S) nanocatalysts with exposed (100)- and (010)-type facets for flat and zigzag planes, respectively. The exposed facet densities of the nanocatalysts are varied via their aspect ratios. Nanocrystals with highly exposed (010)-type surfaces exhibit the best nitrogen reduction kinetics. Density functional theory calculation reveals that the protruded Cu and S atomic arrangement on the zigzag (010)-type surface promotes N2 adsorption and facilitates proton transfer from near the S site to *N2 at the Cu site, thus fast-forwarding electrochemical nitrogen reduction.
https://pubs.acs.org/doi/10.1021/acscatal.2c03680?cookieSet=1
 Flattening bent Janus nanodiscs expands lattice parameters
Flattening bent Janus nanodiscs expands lattice parameters
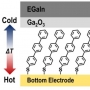
 High Seebeck Coefficient Achieved by Multinuclear Organometal...
High Seebeck Coefficient Achieved by Multinuclear Organometal...