김지현(제1저자, 석박사통합과정 8학기)
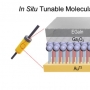
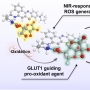
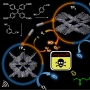
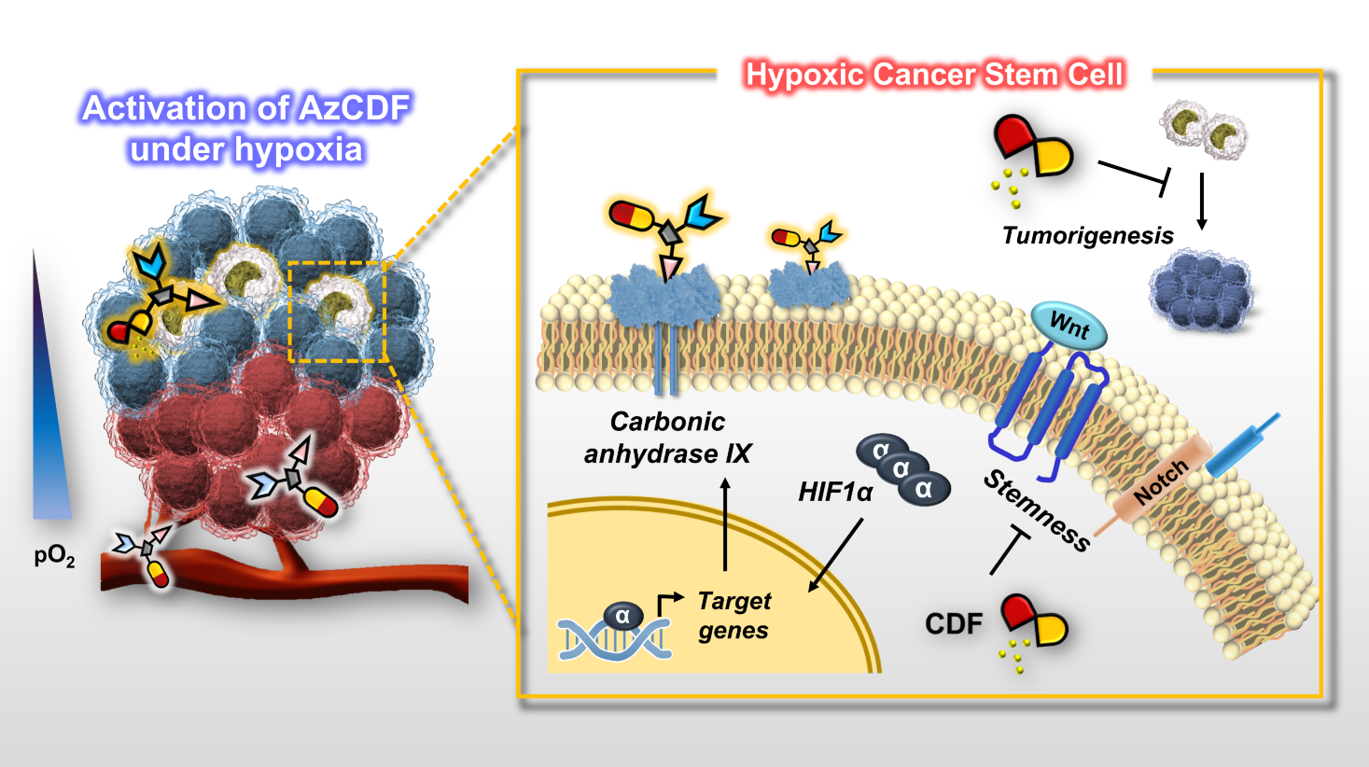
Breast cancer consists of heterogenic subpopulations, which determine the prognosis and response to chemotherapy. Among these subpopulations, a very limited number of cancer cells are particularly problematic. These cells, known as breast cancer stem cells (BCSCs), are thought responsible for metastasis and recurrence. They are thus major contributor to the unfavorable outcomes seen for many breast cancer patients. BCSCs are more prevalent in the hypoxic niche. This is an oxygen-deprived environment that is considered crucial to their proliferation, stemness, and self-renewal but also one that makes BCSCs highly refractory to traditional chemotherapeutic regimens. Here we report a small molecule construct, AzCDF, that allows the therapeutic targeting of BCSCs and which is effective in normally refractory hypoxic tumor environments. A related system, AzNap, has been developed that permits CSC imaging. Several design elements are incorporated into AzCDF, including the CAIX inhibitor acetazolamide (Az) to promote localization in MDA-MB-231 CSCs, a dimethylnitrothiophene subunit as a hypoxia trigger, and a 3,4-difluorobenzylidene curcumin (CDF) as a readily released therapeutic payload. This allows AzCDF to serve as a hypoxia-liable molecular platform that targets BCSCs selectively which decreases CSC migration, retards tumor growth, and lowers tumorigenesis rates as evidenced by a combination of in vitro and in vivo studies. To the best of our knowledge, this is the first time a CSC-targeting small molecule has been shown to prevent tumorigenesis in an animal model.

https://pubs.acs.org/doi/10.1021/jacs.1c03875
 Mechanical Force for the Transformation of Aziridine into Imine
Mechanical Force for the Transformation of Aziridine into Imine
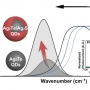
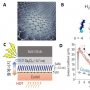
 Mitochondria-targeted nanotheranostic: Harnessing single-lase...
Mitochondria-targeted nanotheranostic: Harnessing single-lase...