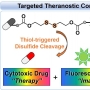
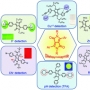
Theranostics, chemical entities designed to combine therapeutic effects and imaging capability within one molecular system, have received considerable attention in recent years. Much of this interest reflects the promise inherent in personalized medicine, including disease-targeted treatments for cancer patients. One important approach to realizing this latter promise involves the development of so-called theranostic conjugates, multicomponent constructs that selectively target cancer cells and deliver cytotoxic agents while producing a readily detectable signal that can be monitored both in vitro and in vivo. This requires the synthesis of relatively complex systems comprising imaging reporters, masked chemotherapeutic drugs, cleavable linkers, and cancer targeting ligands. Ideally, the cleavage process should take place within or near cancer cells and be activated by cellular components that are associated with cancer states or specifically expressed at a higher level in cancer cells. Among the cleavable linkers currently being explored for the construction of such localizing conjugates, disulfide bonds are particularly attractive. This is because disulfide bonds are stable in most blood pools but are efficiently cleaved by cellular thiols, including glutathione (GSH) and thioredoxin (Trx), which are generally found at elevated levels in tumors. When disulfide bonds are linked to fluorophores, changes in emission intensity or shifts in the emission maxima are typically seen upon cleavage as the result of perturbations to internal charge transfer (ICT) processes. In well-designed systems, this allows for facile imaging. In this Account, we summarize our recent studies involving disulfide-based fluorescent drug delivery conjugates, including preliminary tests of their biological utility in vitro and in vivo.
To date, a variety of chemotherapeutic agents, such as doxorubicin, gemcitabine, and camptothecin, have been used to create disulfide-based conjugates, as have a number of fluorophores, including naphthalimide, coumarin, BODIPY, rhodol, and Cy7. The resulting theranostic core (drug−disulfide−fluorophore) can be further linked to any of several site-localizing entities, including galactose, folate, biotin, and the RGD (Arg-Gly-Asp) peptide sequence, to create systems with an intrinsic selectivity for cancer cells over normal cells. Site-specific cleavage by endogenous thiols serves to release the cytotoxic drug and produce an easy-to-monitor change in the fluorescence signature of the cell. On the basis of the results summarized in this Account, we propose that disulfide-based cancer-targeting theranostics may have a role to play in advancing drug discovery efforts, as well as improving our understanding of cellular uptake and drug release mechanisms.

http://pubs.acs.org/doi/abs/10.1021/acs.accounts.5b00406
 Supramolecular Enhancement of Protein Analysis via the Recogn...
Supramolecular Enhancement of Protein Analysis via the Recogn...
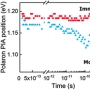 Spectroscopically tracking charge separation in polymer : ful...
Spectroscopically tracking charge separation in polymer : ful...