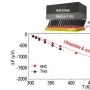
Ammonia is a promising energy vector that can store the high energy density of hydrogen. For this reason, numerous adsorbents have been investigated as ammonia storage materials, but ammonia adsorbents with a high gravimetric/volumetric ammonia capacity that can be simultaneously regenerated in an energy-efficient manner remain underdeveloped, which hampers their practical implementation. Herein, we report Ni_acryl_TMA (TMA = thiomallic acid), an acidic group-functionalized metal-organic framework prepared via successive post-synthetic modifications of mesoporous Ni2Cl2BTDD (BTDD = bis(1H-1,2,3,-triazolo [4,5-b],-[4',5'-i]) dibenzo[1,4]dioxin). By virtue of the densely located acid groups, Ni_acryl_TMA exhibited a top-tier gravimetric ammonia capacity of 23.5 mmol g-1 and the highest ammonia storage of 0.39 g cm-3 at 1 bar and 298 K. The structural integrity and ammonia storage capacity of Ni_acryl_TMA were maintained after ammonia adsorption-desorption tests over five cycles. Temperature-programmed desorption analysis revealed that the moderate strength of the interaction between the functional groups and ammonia significantly reduced the desorption temperature compared to that of the pristine framework with open metal sites. The structures of the post-synthetic modified analogs were elucidated based on Pawley/Rietveld refinement of the synchrotron powder X-ray diffraction patterns and van der Waals (vdW)-corrected density functional theory (DFT) calculations. Furthermore, the ammonia adsorption mechanism was investigated via in-situ infrared and vdW-corrected DFT calculations, revealing an atypical guest-induced binding mode transformation of the integrated carboxylate. Dynamic breakthrough tests showed that Ni_acryl_TMA can selectively capture traces of ammonia under both dry and wet conditions (80% relative humidity). These results demonstrate that Ni_acryl_TMA is a superior ammonia storage/capture material.
DOI: https://doi.org/10.1021/jacs.2c01117
 Functionalization of Diamine-Appended MOF-Based Adsorbents by...
Functionalization of Diamine-Appended MOF-Based Adsorbents by...
 Li-ion Intercalation, Rectification, and Solid Electrolyte In...
Li-ion Intercalation, Rectification, and Solid Electrolyte In...