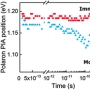
The asymmetric total syntheses of deshydroxy-tetramethylcupressuflavone and desmethylkotanin and the synthesis of a key intermediate in the previous synthesis of hibarimicinone were described using an axially chiral biaryl boronic acid, prepared by the diastereomeric resolution of the corresponding rac-boronic acid, as a common intermediate. Conversion of the boronic acid moiety into either a hydrogen atom or a hydroxy group by protodeboronation or oxidation enabled us to complete the total syntheses of deshydroxytetramethylcupressuflavone and desmethylkotanin and synthesis of a key intermediate in the previous total synthesis of hibarimicinone, respectively.
http://onlinelibrary.wiley.com/doi/10.1002/adsc.201500798/abstract
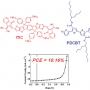
 A High Efficiency Nonfullerene Organic Solar Cell with Optimi...
A High Efficiency Nonfullerene Organic Solar Cell with Optimi...
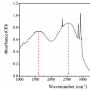
 Origin of the Reversible Thermochromic Properties of Polydiac...
Origin of the Reversible Thermochromic Properties of Polydiac...