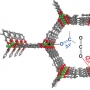
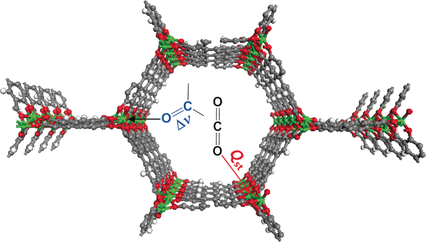
A series of metal–organic frameworks (MOFs) M2(dobpdc) (M=Mn, Co, Ni, Zn; H4dobpdc=4,4′-dihydroxy-1,1′-biphenyl-3,3′-dicarboxylic acid), with a highly dense arrangement of open metal sites along hexagonal channels were prepared by microwave-assisted or simple solvothermal reactions. The activated materials were structurally expanded when guest molecules including CO2 were introduced into the pores. The Lewis acidity of the open metal sites varied in the order Mn<Co<Ni>Zn, as confirmed by C=O stretching bands in the IR spectra, which are related to the CO2 adsorption enthalpy. DFT calculations revealed that the high CO2 binding affinity of transition-metal-based M2(dobpdc) is primarily attributable to the favorable charge transfer from CO2(oxygen lone pair acting as a Lewis base) to the open metal sites (Lewis acid), while electrostatic effects, the underlying factor responsible for the particular order of binding strength observed across different transition metals, also play a role. The framework stability against water coincides with the order of Lewis acidity. In this series of MOFs, the structural stability of Ni2(dobpdc) is exceptional; it endured in water vapor, liquid water, and in refluxing water for one month, and the solid remained intact on exposure to solutions of pH 2–13. The DFT calculations also support the experimental finding that Ni2(dobpdc) has higher chemical stability than the other frameworks.
http://onlinelibrary.wiley.com/doi/10.1002/chem.201600189/full
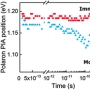
 Investigation of Charge Carrier Behavior in High Performance ...
Investigation of Charge Carrier Behavior in High Performance ...
 Programmed activation of cancer cell apoptosis: A tumor-targe...
Programmed activation of cancer cell apoptosis: A tumor-targe...