“Inorganic Chemistry in Proteinaceous Environments”
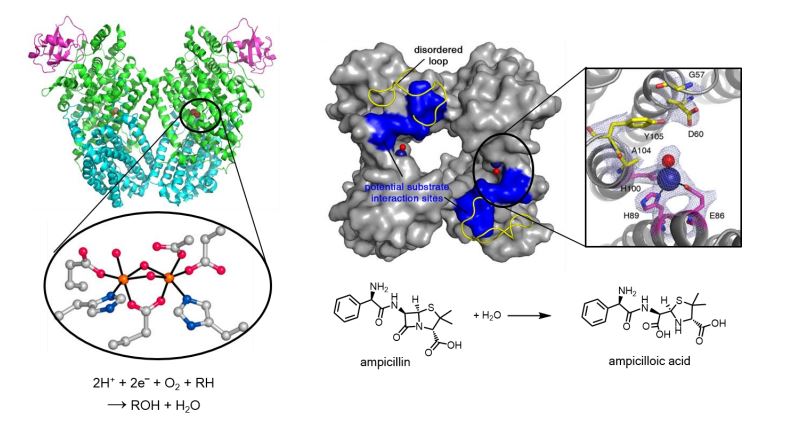
Natural enzymes exhibit high catalytic efficiency and selectivity for their dedicated chemical transformations, and the power of enzyme catalysis has enticed chemists to scrutinize and mimic the chemical mechanism and reactivities. Particularly, a third of natural enzymes are found with metal ions, suggesting that inorganic elements are crucial in biological environments. From the studies of a natural enzyme, toluene/o-xylene monooxygenase hydroxylase, which belongs to the family of carboxylatebridged diiron proteins, we demonstrated that the diiron active site generates a catalytically reactive intermediate during dioxygen activation, and the protein environment functions as a sophisticatedly programmed matrix that regulates overall catalytic process. Inspired by the beauty of biochemical architectures associated with metal ions, we have designed a protein that self-assembles into a tetramer via hydrophobic interactions, disulfide bonds, and zinc coordination. The artificial protein yields catalytically active zinc-binding sites, which has been further optimized for molecular interactions with a substrate, ampicillin, by directed evolution. We have learned that design of artificial metalloenzyme can be considered as a distinctive route of reproducing and developing orchestrated chemical/biological events, and we suppose that protein engineering can aid us to develop efficient and sustainable biocatalysts and biomaterials in conjunction with synthetic biology, bioinformatics, and other related fields.
 반도체시스템공학과 초청세미나 안내(SK하이닉스 이상선 부사장)
반도체시스템공학과 초청세미나 안내(SK하이닉스 이상선 부사장)