Tae Su Choi(Post doc.)
Regulation of amyloid-β (Aβ) aggregation by metal ions and proteins is
essential for
understanding the pathology of Alzheimer’s disease (AD). Human serum albumin
(HSA), a regulator of metal and protein transportation, can modulate metal–Aβ interactions and Aβ aggregation in human fluid; however, the
molecular mechanisms for such activities remain unclear. Herein, we report the
molecular-level complexation between Zn(II), Cu(II), Aβ, and HSA, which is able to alter
the aggregation and cytotoxicity of Aβ peptides and induce their cellular
transportation. In addition, a single Aβ monomer
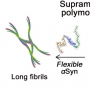
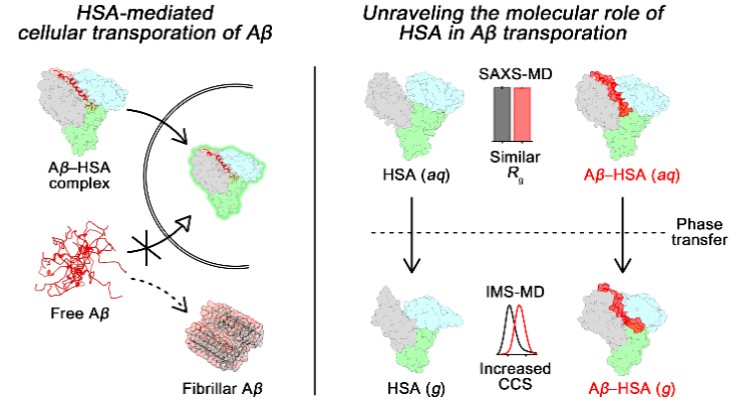
bound to HSA is observed with the structural change of Aβ from a random coil to an α-helix.
Small-angle X-ray scattering (SAXS) studies indicate that Aβ–HSA complexation causes no structural
variation of HSA in solution. Conversely, ion mobility mass spectrometry
(IM-MS) results present that Aβ prevents the shrinkage of the
V-shaped groove of HSA in the gas phase. Consequently, for the first time, HSA is demonstrated to predominantly capture a
single Aβ monomer at the groove using the
phase transfer of a protein heterodimer from solution to the gas phase.
Moreover, HSA sequesters Zn(II) and Cu(II) from Aβ
while maintaining HSA–Aβ interaction.
Therefore, HSA is capable
of controlling metal-free and metal-bound Aβ
aggregation and aiding the cellular transportation of Aβ via Aβ–HSA complexation. The overall
results and observations regarding HSA, Aβ, and metal ions
advance our knowledge of how protein-protein interactions associated with Aβ and metal ions could be linked to AD pathogenesis.
http://pubs.acs.org/doi/10.1021/jacs.7b08584
 Synthesis, Characterization, and Efficient Catalytic Activiti...
Synthesis, Characterization, and Efficient Catalytic Activiti...
 Two Regioisomeric π-Conjugated Small Molecules: Synthesis, Ph...
Two Regioisomeric π-Conjugated Small Molecules: Synthesis, Ph...