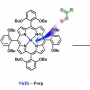
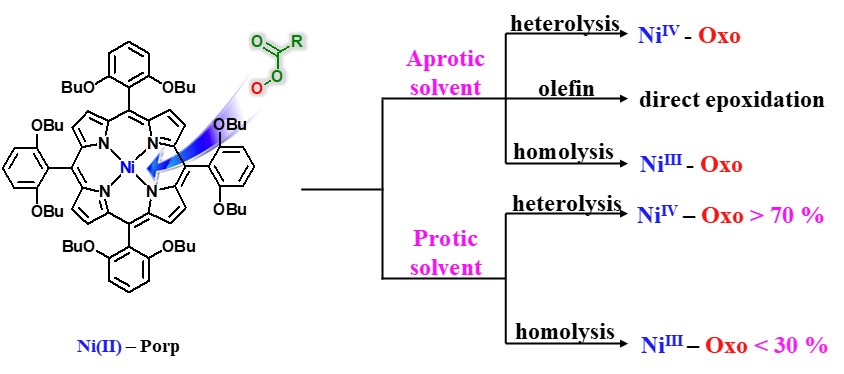
A new nickel(II) porphyrin complex, [NiII(porp)] (1), has been synthesized and characterized by 1H NMR, 13C NMR and mass spectrometry analysis. This NiII porphyrin complex 1 quantitatively catalyzed the epoxidation reaction of a wide range of olefins with meta-chloroperoxybenzoic acid (m-CPBA) under mild conditions. Reactivity and Hammett studies, H218O-exchange experiments, and the use of PPAA (peroxyphenylacetic acid) as a mechanistic probe suggested that participation of multiple active oxidants NiII−OOC(O)R 2, NiIV-Oxo 3, and NiIII-Oxo 4 within olefin epoxidation reactions by the nickel porphyrin complex is markedly affected by solvent polarity, concentration, and type of substrate. In aprotic solvent systems, such as toluene, CH2Cl2, and CH3CN, multiple oxidants, NiII−(O)R 2, NiIV-Oxo 3, and NiIII-Oxo 4, operate simultaneously as the key active intermediates responsible for epoxidation reactions of easy-to-oxidize substrate cyclohexene, whereas NiIV-Oxo 3 and NiIII-Oxo 4 species become the common reactive oxidant for the difficult-to-oxidize substrate 1-octene. In a protic solvent system, a mixture of CH3CN and H2O (95:5), the NiII−OOC(O)R 2 undergoes heterolytic or homolytic O−O bond cleavage to afford NiIV-Oxo 3and NiIII-Oxo 4 species by general acid catalysis prior to direct interaction between 2 and olefin, regardless of the type of substrate. In this case, only NiIV-Oxo 3 and NiIII-Oxo 4 species were the common reactive oxidant responsible for olefin epoxidation reactions.
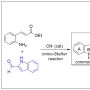 Total Syntheses of Arcyriaflavin A and Calothrixin B Using 2,...
Total Syntheses of Arcyriaflavin A and Calothrixin B Using 2,...
 Molecular Insights into Human Serum Albumin as a Receptor of ...
Molecular Insights into Human Serum Albumin as a Receptor of ...