Sungjong Lee(석사과정) / Kyung-Hee Kim(박사과정)
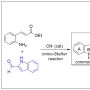
A new protocol for the synthesis of 2,2′-bisindole-3-acetic acid derivatives from aldimines derived from 2-aminocinnamic acid derivatives and indole-2-carboxaldehyde was developed via a cyanide-catalyzed imino-Stetter reaction. With this protocol, the divergent total syntheses of arcyriaflavin A, a representative indolocarbazole natural product, and calothrixin B, a representative indolo[3,2-j]phenanthridine natural product, were completed using a 2,2′-bisindole-3-acetic acid derivative as the common intermediate.
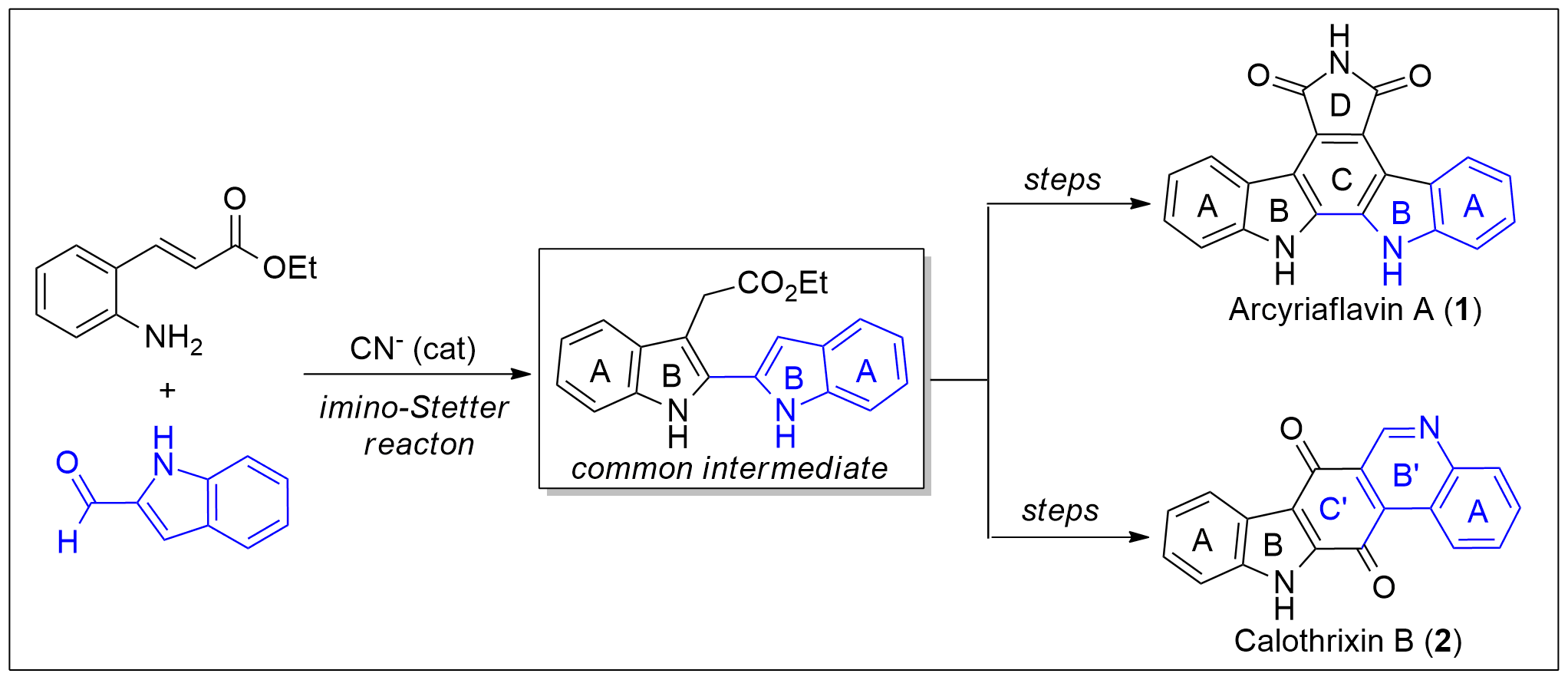
http://pubs.acs.org/doi/full/10.1021/acs.orglett.7b00687
 Collision Cross Sections and Ion Structures: Development of a...
Collision Cross Sections and Ion Structures: Development of a...
 Synthesis, Characterization, and Efficient Catalytic Activiti...
Synthesis, Characterization, and Efficient Catalytic Activiti...