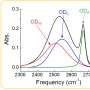
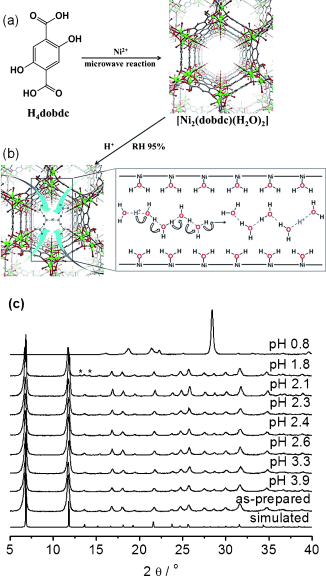
A porous metal–organic framework (MOF), [Ni2(dobdc)(H2O)2]⋅6 H2O (Ni2(dobdc) or Ni-MOF-74; dobdc4−=2,5-dioxido-1,4-benzenedicarboxylate) with hexagonal channels was synthesized using a microwave-assisted solvothermal reaction. Soaking Ni2(dobdc) in sulfuric acid solutions at different pH values afforded new proton-conducting frameworks, H+@Ni2(dobdc). At pH 1.8, the acidified MOF shows proton conductivity of 2.2×10−2 S cm−1 at 80 °C and 95 % relative humidity (RH), approaching the highest values reported for MOFs. Proton conduction occurs via the Grotthuss mechanism with a significantly low activation energy as compared to other proton-conducting MOFs. Protonated water clusters within the pores of H+@Ni2(dobdc) play an important role in the conduction process.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201404164/full
 Diamine-functionalized metal–organic framework: exceptionally...
Diamine-functionalized metal–organic framework: exceptionally...
 Template-Guided Solution-Shearing Method for Enhanced Charge ...
Template-Guided Solution-Shearing Method for Enhanced Charge ...