임동준
(제1저자, 통합과정생)
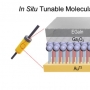
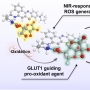
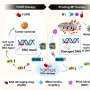
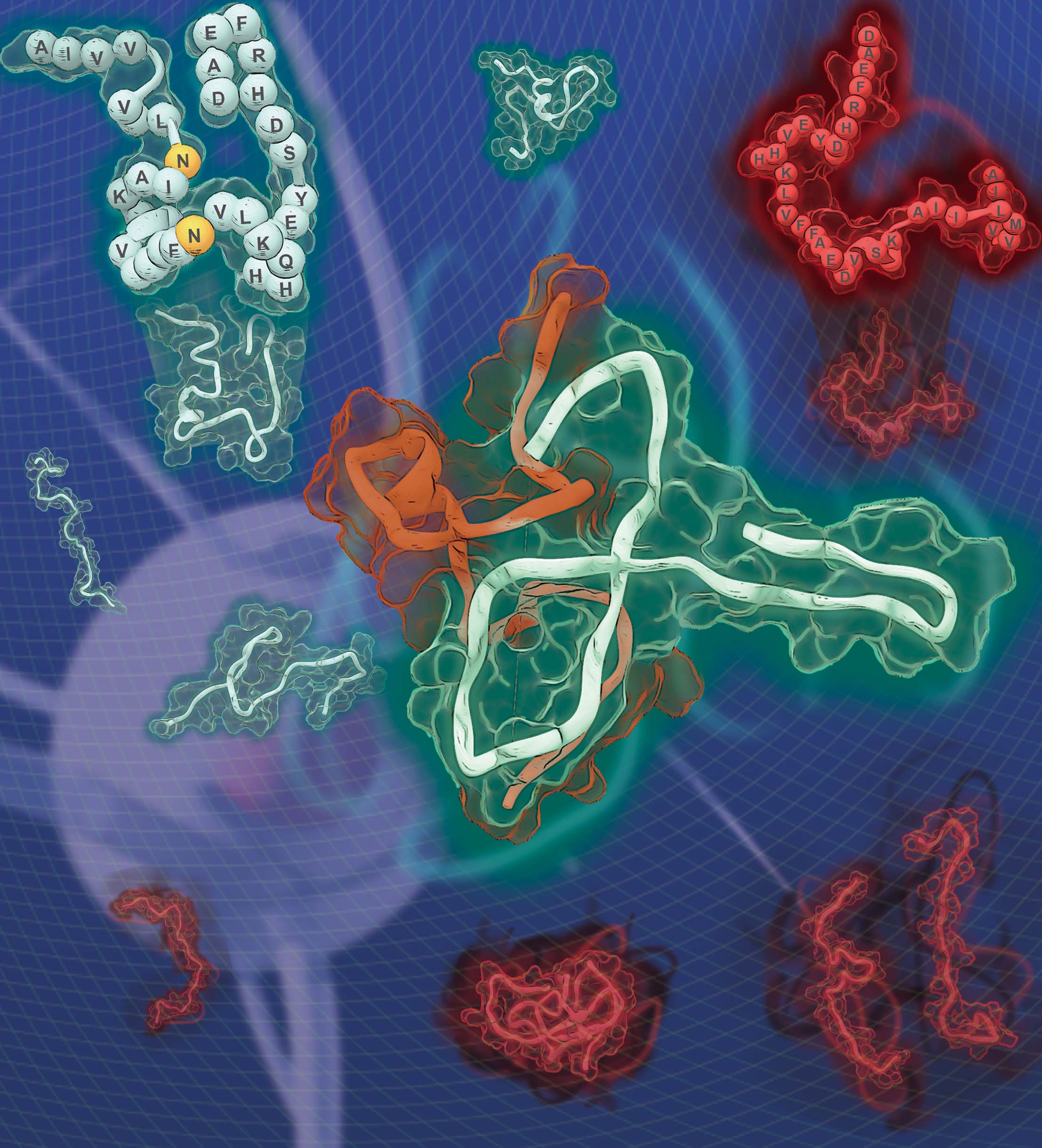
Several point mutations can modulate protein structure and dynamics, leading to different natures. Especially in the case of amyloidogenic proteins closely related to neurodegenerative diseases, structural changes originating from point mutations can affect fibrillation kinetics. Herein, we rationally designed mutant candidates to inhibit the fibrillation process of amyloid-β with its point mutants through multistep in silico analyses. Our results showed that the designed mutants induced kinetic self-assembly suppression and reduced the toxicity of the aggregate. A multidisciplinary biophysical approach with small-angle X-ray scattering, ion mobility-mass spectrometry, mass spectrometry, and additional in silico experiments was performed to reveal the structural basis associated with the inhibition of fibril formation. The structure-based design of the mutants with suppressed self-assembly performed in this study could provide a different perspective for modulating amyloid aggregation based on the structural understanding of the intrinsically disordered proteins.
https://pubs.acs.org/doi/10.1021/jacs.1c10173
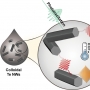 Midwavelength Infrared Colloidal Nanowire Laser
Midwavelength Infrared Colloidal Nanowire Laser
 Safeguarding the RuO2 phase against lattice oxygen oxidation ...
Safeguarding the RuO2 phase against lattice oxygen oxidation ...