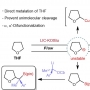
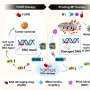
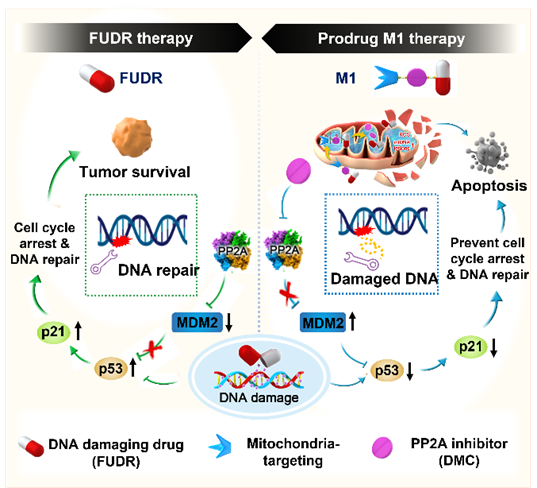
We report a novel multifunctional construct, M1, designed explicitly to target the DNA damage response in cancer cells. M1 contains both a floxuridine (FUDR) and protein phosphatase 2A (PP2A) inhibitor combined with a GSH-sensitive linker. Further conjugation of the triphenylphosphonium moiety allows M1 to undergo specific activation in the mitochondria, where mitochondria-mediated apoptosis is observed. Moreover, M1 has enormous effects on genomic DNA ascribed to FUDR's primary function of impeding DNA/RNA synthesis combined with diminishing PP2A-activated DNA repair pathways. Importantly, mechanistic studies highlight the PP2A obtrusion in FUDR/5-fluorouracil (5-FU) therapy and underscore the importance of its inhibition to harbor therapeutic potential. HCT116 cell xenograft-bearing mice that have a low response rate to 5-FU show a prominent effect with M1, emphasizing the importance of DNA damage response targeting strategies using tumor-specific microenvironment-activatable systems.
https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202117075
 Harnessing GLUT1-Targeted Pro-oxidant Ascorbate for Synergist...
Harnessing GLUT1-Targeted Pro-oxidant Ascorbate for Synergist...
 Midwavelength Infrared Colloidal Nanowire Laser
Midwavelength Infrared Colloidal Nanowire Laser