9월22일(목)대학원세미나 Prof. Anna Lee(Myongji University, Department of Chemistry, Yongin, Korea)
| 초청강사 | Prof. Anna Lee |
|---|---|
| 소속 | Myongji University, Department of Chemistry, Yongin, Korea |
| 일시 | 2016년 9월 22일(목) 오후5시 |
| 장소 | 아산이학관 331 |
“Organocatalyzed Asymmetric Reactions”
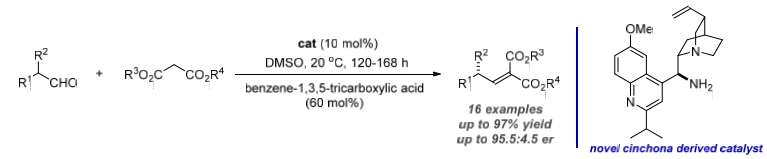
The Catalytic Asymmetric Knoevenagel Condensation: The Knoevenagel condensation reaction developed by Emil Knoevenagel in 1896 is a powerful and efficient carbon-carbon bond forming reaction, but also the root of aminocatalysis. Remarkably though, while the Knoevenagel reaction, as the historic basis of all aminocatalyzed processes, has been incorporated into asymmetric organocascades and domino reactions, and even chiral auxiliary derived malonates have been studied, a variation in which the Knoevenagel reaction itself is utilized to establish asymmetry remained elusive. The main problems of the reaction were reactivity and stereoselectivity issues as well as the formation of by-products. The goal of this work was to develop the first enantioselective Knoevenagel condensation reaction using a novel chiral amine catalyst based on a dynamic kinetic resolution (DKR). Various chiral amine catalysts were synthesized, and we found that modified cinchona based amine catalysts provided the desired product in high yield and enantioselectivity.
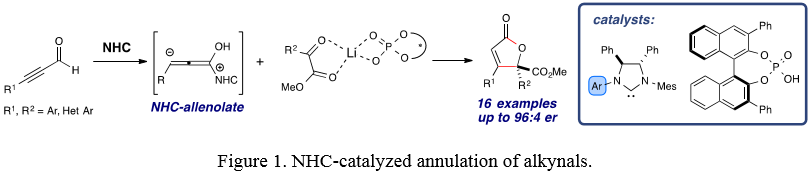
A Cooperative N-Heterocyclic Carbene/Chiral phosphate Catalysis System for Allenolate Annulations: NHC-catalyzed homoenolate additions to various electrophiles represent an enabling class of transformations that have been employed for the construction of complex hetero- and carbocyclic system through unconventional Umpolung reactivity. Recently, our group developed Lewis acid/NHC cooperative catalysis to develope novel transformations. In this study, we have extended the scope of cooperative catalysis by employing chiral phosphoric acids for the first time and this novel cooperative catalysis was applied to the challenging allenolate annulation reaction (Figure 1). A limited number of reports of NHC-catalyzed homoenolate additions involving alkynyl aldehydes have appeared. We have developed a highly efficient asymmetric [3+2] annulation reaction of alkynyl aldehydes with α-ketoesters via NHC/chiral phosphate cooperative catalysis.

References
1. Lee, A.; Michrowska, A.; S.-Mosse, S.; List, B., Angew. Chem. Int. Ed. 2011, 50, 1707.
2. Lee, A.; Reisinger, C.; List, B., Adv. Synth. Catal. 2012, 354, 1701.
3. Lee, A.; Scheidt, K. A., Angew. Chem. Int. Ed. 2014, 53, 7594.
4. Lee, A.; Younai, A. ; Price, C. K. ; Izquierdo, J. ; Mishra, R. K. ; Scheidt, K. A., J. Am. Chem. Soc. 2014, 136, 10589.
5. Lee, A.; Scheidt, K. A., Chem. Commun. 2015, 51, 3407.